>
<
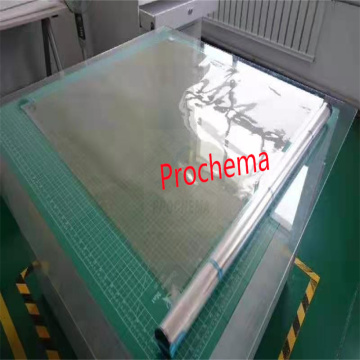
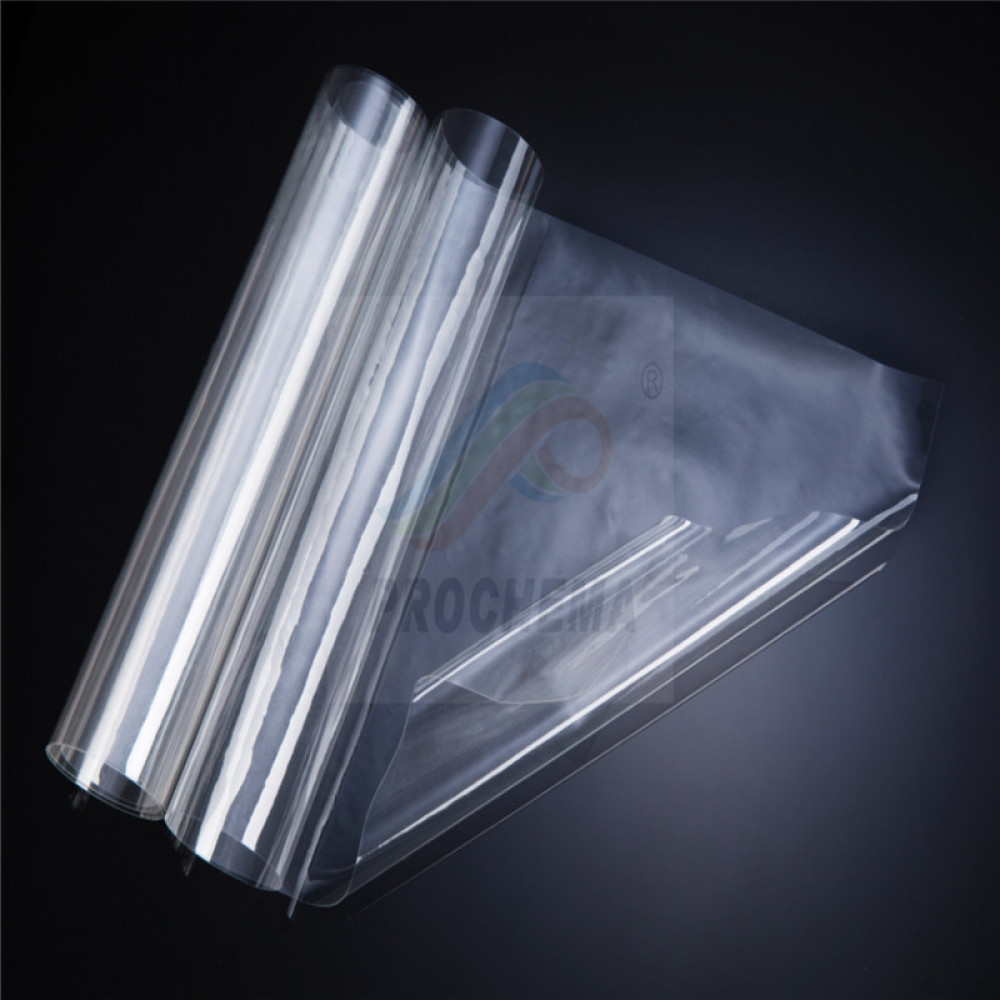
Application of PFEM PEM in Vanadium Battery Industry
Price:
-
$350.00≥5 Square Meter

Mianyang Prochema Commercial Co.,Ltd.

22 YRS
Mianyang, Sichuan, China
Business type:Manufacturer,Trade Company,Distributor/Wholesaler
Close
Basic Info
Place of Origin
China
Supply Ability
10000M2/MONTH
Payment Type
L/C,T/T,D/P,Paypal,D/A
Incoterm
FOB,DDU,CFR,Express Delivery,CIF,EXW,DAF,FAS,DES,FCA,CPT,CIP,DEQ,DDP
Certificate
ISO
HS Code
3921909001
Transportation
Ocean,Land,Air,Express
Port
SHANGHAI,GUANGZHOU,SHENZHEN
View All Details
Product Description
The Application of Perfluorinated Ion Exchange Membrane in Vanadium Battery Industry
Perfluorinated ion exchange membrane (PFEM) has been widely used in the vanadium battery industry due to its excellent chemical stability, high ion conductivity, and good mechanical properties. Vanadium batteries are a type of rechargeable flow battery that store energy by utilizing the redox reactions of vanadium ions in solution. PFEM plays a crucial role in separating the positive and negative electrolytes, allowing the flow of ions while preventing the mixing of the electrolytes.
One of the main advantages of PFEM is its high chemical stability. It is resistant to a wide range of corrosive substances, including strong acids and bases, making it suitable for use in vanadium battery systems where the electrolytes are highly acidic. This stability ensures the longevity and reliability of the battery system.
PFEM also exhibits high ion conductivity, allowing for efficient transfer of vanadium ions between the positive and negative electrolytes. The high ion conductivity contributes to the overall performance of the battery, enabling fast charging and discharging rates.
Furthermore, PFEM possesses good mechanical properties, such as flexibility and durability. These properties ensure that the membrane can withstand the mechanical stress and pressure changes during the operation of the vanadium battery system. The durability of PFEM allows for long-term use without significant degradation, ensuring the stability and efficiency of the battery system.
In conclusion, the application of perfluorinated ion exchange membrane in the vanadium battery industry has greatly contributed to the development of efficient and reliable energy storage systems. Its chemical stability, high ion conductivity, and good mechanical properties make it an ideal choice for separating the electrolytes and facilitating the redox reactions in vanadium batteries.
Perfluorinated ion exchange membrane (PFEM) has been widely used in the vanadium battery industry due to its excellent chemical stability, high ion conductivity, and good mechanical properties. Vanadium batteries are a type of rechargeable flow battery that store energy by utilizing the redox reactions of vanadium ions in solution. PFEM plays a crucial role in separating the positive and negative electrolytes, allowing the flow of ions while preventing the mixing of the electrolytes.
One of the main advantages of PFEM is its high chemical stability. It is resistant to a wide range of corrosive substances, including strong acids and bases, making it suitable for use in vanadium battery systems where the electrolytes are highly acidic. This stability ensures the longevity and reliability of the battery system.
PFEM also exhibits high ion conductivity, allowing for efficient transfer of vanadium ions between the positive and negative electrolytes. The high ion conductivity contributes to the overall performance of the battery, enabling fast charging and discharging rates.
Furthermore, PFEM possesses good mechanical properties, such as flexibility and durability. These properties ensure that the membrane can withstand the mechanical stress and pressure changes during the operation of the vanadium battery system. The durability of PFEM allows for long-term use without significant degradation, ensuring the stability and efficiency of the battery system.
In conclusion, the application of perfluorinated ion exchange membrane in the vanadium battery industry has greatly contributed to the development of efficient and reliable energy storage systems. Its chemical stability, high ion conductivity, and good mechanical properties make it an ideal choice for separating the electrolytes and facilitating the redox reactions in vanadium batteries.
Q: What's your supportive policy for distributors in overseas market?
A:We support in many aspects including marketing, promotion, product developmentimprovements
You May Also LikeRelated Keywords
You May Also Like
>
<
Other popular products
>
<
Contact Now